Pitting and crevice corrosion of offshore stainless steel tubing
Gerhard Schiroky
Swagelok Co.
Anibal Dam
BP Exploration & Production Inc.
Akinyemi Okeremi
Shell International Exploration & Production
Charlie Speed
Consultant
Oil and gas platforms regularly use stainless steel tubing in process instrumentation and sensing, as well as in chemical inhibition, hydraulic lines, impulse lines, and utility applications, over a wide range of temperatures, flows, and pressures. Corrosion of 316 stainless steel tubing has been observed in offshore applications around the world. Corrosion is a serious development that can lead to perforations of the tubing wall and the escape, under pressure, of highly flammable chemicals.
The two prevalent forms of localized corrosion are pitting, often readily recognizable, and crevice, which can be more difficult to see. Many factors contribute to the onset of localized corrosion. Inadequate tubing alloy and suboptimal installation practices can lead to deterioration of tubing surfaces in a matter of months. It is speculated that today's minimally alloyed 316 stainless steel tubing, with about 10% nickel, 2% molybdenum, and 16% chromium, may more readily corrode than the more generously alloyed 316 tubing products produced decades ago.
Contamination is another leading cause for surface degradation. Such contamination may be caused by iron particles from welding and grinding operations; surface deposits from handling, drilling, and blasting; and from sulfur-rich diesel exhaust. Periodic testing of seawater deluge systems, especially in combination with insufficient freshwater cleansing, may leave undesirable chloride-laden deposits behind.
Pitting and crevice corrosion
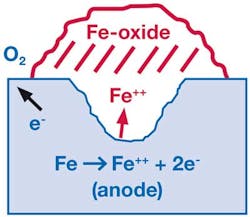
Pitting corrosion of tubing usually is readily recognized. Individual shallow pits, and in later stages, deep and sometimes connected pits can be seen with the unaided eye. Pitting corrosion starts when the chromium-rich passive oxide film on 316 tubing breaks down in a chloride-rich environment. The higher the chloride concentration and the more elevated the temperature, the more likely the breakdown of this passive film.
Once the passive film is breached, an electrochemical cell becomes active. Iron goes into solution in the more anodic bottom of the pit, diffuses toward the top, and oxidizes to iron oxide. The concentration of the iron chloride solution in a pit can increase as the pit deepens. The consequences are accelerated pitting, perforation of tubing walls and leaks. Pitting can penetrate deep into the tubing walls, creating a situation where tubing could fail.
Crevices are difficult, or even impossible, to avoid in tubing installations. They exist between tubing and tube supports, in tubing clamps, between adjacent tubing runs, and underneath contamination and deposits that may accumulate on tubing surfaces. Relatively tight crevices pose the greatest danger. General corrosion of tubing in a tight crevice causes the oxygen concentration in the fluid that is contained within a crevice to drop. A lower oxygen concentration increases the likelihood for breakdown of the passive surface oxide film. The result is a shallow pit.
Unlike in pitting corrosion, formation of a pit on tubing surrounded by a crevice leads to an increase of the Fe++ concentration in the fluid contained in the gap. Because of the strong interaction of the Fe++ ions with the OH ions, the pH value drops. Chloride ions also will diffuse into the gap, being attracted by the Fe++ ions. The result is an acidic ferric chloride solution that can accelerate corrosion of tubing within the crevice.
Ideally, tubing should resist all forms of corrosion, including general, localized (pitting and crevice), galvanic, microbiological, chloride-induced stress corrosion cracking, and sour gas cracking. The tubing also should have adequate mechanical properties, especially when fluid pressures are high. Resistance to erosion comes into play when fluids contain potentially erosive particles. The environmental impact of the tubing also should be a concern; aquatic life can be harmed by small concentrations of copper ions that can be released by copper-zinc alloys.
The resistance of an alloy to localized tubing corrosion can be estimated from its chemical composition by calculating the alloy's pitting resistance equivalent number (PREN). The most frequently used relationship is: PREN = %Cr + 3.3 %Mo + 16 %N. The higher the PREN value of an alloy, the higher its resistance to localized corrosion, i.e., the higher its critical pitting temperature (CPT) and critical crevice corrosion temperature (CCT). These critical temperatures can be determined by common testing procedures such as ASTM G48 and ASTM G150.
Alloy selection
The importance of selecting the optimal alloy is demonstrated when austenitic 316 stainless steel tubing shows heavy corrosion while no signs of corrosion were detected on alloy 2507 superduplex tubing installed side by side. In a Gulf of Mexico installation of alloy 2507 tubing, only a few instances of external chloride crevice corrosion damage were identified. No perforations leading to the loss of containment of system fluids were observed. The only instances where crevice corrosion damage occurred involved the use of plastic support strips and neoprene gaskets.
Numerous alloys have been used or have presented themselves as candidates for use in installations that require resistance to seawater corrosion. The most frequently used alloys have been the 300-series austenitic stainless steels, mainly 316 and in some cases 317. Alloys with at least 6% molybdenum, the so-called "6-moly" alloys, perform well offshore. Typical 6-moly alloys include 254SMO, AL6XN, and 25-6Mo.
More recently, alloys with slightly more than 6% molybdenum have been introduced: 654SMO, AL6XN Plus, 27-7Mo, and 31. The published properties of these alloys suggest that they would perform well in chloride environments.
Nickel alloys such as 825, 625, and C-276 are more frequently used in sour gas applications. Of these alloys, 625 and C-276 demonstrate excellent resistance to localized corrosion. Ferritic alloys like Sea-Cure and AL29-4C resist attack by aqueous chloride solutions and are primarily used as heat exchanger tubing.
Tubing alloys are available that offer a combination of attractive properties for even unique applications in global construction projects. It is good practice to select an alloy with a critical pitting temperature above operating temperature. Depending on the application, it may be just as important to select an alloy with a critical crevice corrosion temperature above operating temperature.
Even highly corrosion-resistant tubing can be sacrificed when tubing surfaces are not kept clean. If possible, tubing should be installed following heavy construction activities that would otherwise allow weld splatter and grinding debris to accumulate on tubing. Where adjustments of construction sequences are not possible, tubing should be shielded from contamination, and if contaminated, should be thoroughly cleaned.
The growing number of duplex alloys reflects the increasing use of this promising class of materials. The workhorse 2205 duplex alloy was introduced decades ago. Now there is superduplex alloy 2507, which has performed very well in recent years in more demanding applications that require PREN values of 40 and above. More recently, the hyperduplex alloy 3207 was introduced with an even higher PREN value.
At the low end of alloy content, several lean duplex alloys such as 2101, 2304, and 2003 are candidates for less demanding applications.
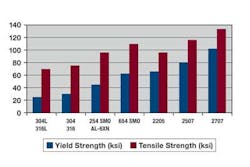
A graph plot of the critical pitting temperature and critical crevice temperature shows the increase in chromium, molybdenum, and nitrogen leads to an increase in the CPT and CCT values of austenitic and duplex stainless steels. That also illustrates the economic advantage of duplex alloys. Despite an overall lower content of costly nickel and molybdenum, they offer performance similar to that of highly alloyed austenitic stainless steels.
Not only do duplex alloys offer satisfactory resistance to localized corrosion, they also have high mechanical properties, which make them prime candidates for high-pressure applications. Note that 2507 has a yield strength more than three times that of 316L.
Jacketed tubing
For applications in seawater, a tubing alloy that is highly resistant to localized corrosion is not the only option. Alternatively, one may select a less resistant alloy and then shield or protect the tubing.
Adequate protection appears to be offered by a thermoplastic polyurethane jacket that can be cost-effectively extruded onto continuous tubing. Recent installations in the Gulf of Mexico combine this clamping concept with superduplex tubing, and will generate valuable performance data.
An alternate approach uses jacketed tubing. The extrusion of a thermoplastic coating onto tubing is an economically attractive solution. Tubing is typically 316 or 317 stainless steel, and the preferred coating is polyurethane. Limited installations that use urethane jacketed 316 tubing report satisfactory results.
While the jacket must offer reliable protection from corrosive fluids, it must fulfill additional requirements. The jacket must resist impact, abrasion, and degradation by UV-radiation. It must allow tubing to bend, and must allow for cost-effective tubing installation, i.e., removal of the jacket and make-up of tubing connections. Once made up, the connections typically have to be protected from the environment using shrink tubing or tape. Without this protection, seawater access could cause pitting corrosion of exposed tubing or crevice corrosion in the gap between the tubing and the jacket.
Appropriate tubing clamps must be selected and care taken to prevent them from cutting into jackets and sacrificing their protective character. Jacketed tubing also can insulate, or heat and insulate, tubing when system fluids must be kept above ambient temperature.
Tubing supports and clamps
Many types of tubing supports and clamps have been used. Some have led to significant crevice corrosion, especially when tight crevices with large crevice surface areas result in depletion of oxygen so the alloy cannot reform the passive oxide layer. In particular, plastic tubing clamps are prone to inducing crevice corrosion because the plastic deforms around the tubing to create tighter crevices that limit oxygen ingress.
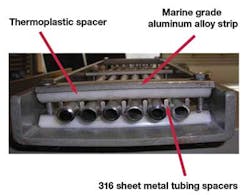
One early approach to preventing or mitigating crevice corrosion was the use of marine aluminum alloys in tubing supports and clamps. The tubing rests on a thin strip of aluminum alloy contained within a fiber-reinforced plastic tray. The tubing is held in place with an aluminum alloy bar.
Tubing support structures that use aluminum alloys appear to perform well. Galvanic corrosion between aluminum alloy and stainless steel may occur, but the aluminum alloy is more anodic than stainless steel, which means aluminum will corrode preferentially. Once sufficient corrosion has taken place over a number of years, affected aluminum supports and clamps can be replaced while the stainless steel tubing remains in place.
An alternate design originally developed for piping supports has recently been adopted for the installation of stainless steel tubing. The tubing is sandwiched between two half-round rods of a thermoplastic material. With the round tubing running perpendicular to the round support rod surface, the crevice contact area is minimized. Theoretically, there should be only one point of contact; however, some plastic deformation of the support rod takes place that results in a finite contact (crevice) area. A benefit of this design is that the supports/clamps allow for differential expansion of tubing and support structure.
Industry standards
The recently published industry standard, NACE SP0108-2008 "Corrosion Control of Offshore Structures by Protective Coatings," provides guidance for more effective corrosion protection for offshore structures. TAnother industry standard, API RP 552 "Transmission Systems," contains a section on installation practices. Those described practices do not address the avoidance of crevice corrosion.